| Absorption | Distribution | Metabolism | Excretion | Special Population | Other Details |
Absorption and Plasma Concentrations
When a single 4 mg dose of silodosin tablet or capsule was administered orally to 27 healthy adult males, respectively, plasma concentrations and pharmacokinetic parameters of silodosin tablet are shown in Table 1 and Figure 1.
It was demonstrated that the silodosin tablet of 4 mg and capsule of 4 mg are biologically equivalent.
Table 1 Pharmacokinetic parameters following postprandial administration of 4 mg oral dose tablet in healthy adult male volunteers (mean±SD)
| Cmax (ng/mL) | AUC0-α a) (ng ∙ hr/mL) | Tmax (hr) | t 1/2 (hr) | |
| No. of subjects | 27 | 27 | 27 | 27 |
| 4 mg tablet | 29.3±13.9 | 122.9±39.3 | 0.9±0.7 | 5.8±3.4 |
| 4 mg capsule | 28.9±14.7 | 125.4±40.1 | 1.3±0.9 | 5.9±4.0 |
| a): In the case of AUC 0-48 hr, AUC 0-10 hr was tabulated as AUC 0-48 hr for 1 subject out of 27 subjects. | ||||
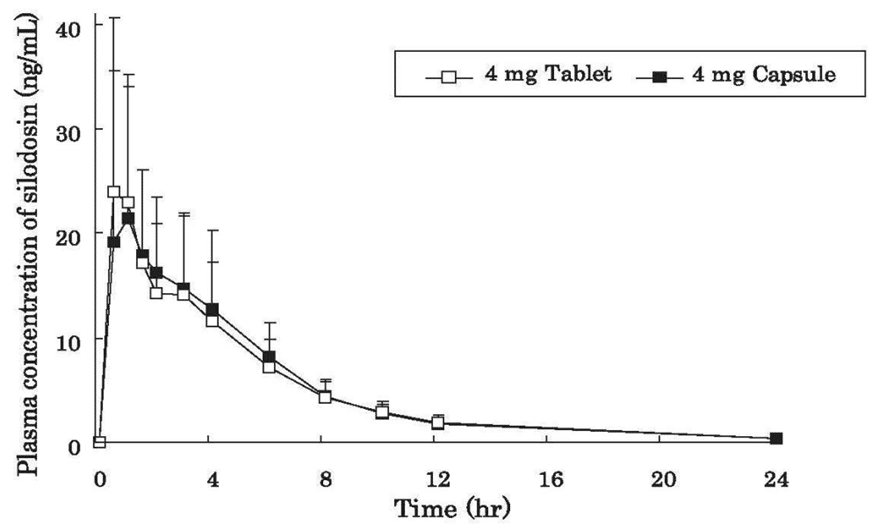
Figure 1 Time course of changes in plasma concentrations of silodosin following a single administration at a dose of 4 mg tablet in healthy adult male volunteers under fasting conditions. Data are expressed as mean± SD (n=27)
When a single oral dose of this drug was administered to healthy adult male volunteers (6 subjects/group) at doses ranging from 0.5 to 12 mg, plasma concentrations of silodosin increased dose-dependently, and Cmax and AUC0-α showed linearity. The time course of changes in plasma concentrations of silodosin following a single oral administration of silodosin at a dose of 2 or 4 mg is shown in Figure 2.

Figure 2 Time course of changes in plasma concentrations of silodosin following a single administration at a dose of 2 or 4 mg in healthy adult male volunteers under fasting conditions. Data are expressed as mean± SD (n=6)
When silodosin was administered orally twice daily for 7 days (once daily on days 1 and 7) at a dose of 4 mg/dose in 5 healthy adult male volunteers, plasma concentrations of silodosin reached a steady state on day 3. The accumulation factor relative to the first dose was 1.1-fold.4) (Table 2)
Table 2 Pharmacokinetic parameters following postprandial administration of 4 mg dose in healthy adult male volunteers (mean± SD)
| Cmax (ng/mL) | AUC0-α a) (ng ∙ hr/mL) | Tmax (hr) | t 1/2 (hr) | |
| Single dose | 26.8 ± 9.2 | 143.9 ± 57.1 | 2.2 ± 0.5 | 6.9 ± 3.1 |
| Repeated dose | 28.7 ± 7.6 | 134.3 ± 39.0 | 2.0 ± 0.0 | 10.4 ± 4.6 |
Pharmacokinetic parameters following repeated administration are shown as the results obtained from the time course of changes in concentration on day 7 less the cumulative concentration from days 0-6.
When a single 4 mg dose of silodosin was administered orally to 12 elderly males (age range: 65 to 75 year) postprandially, no obvious differences in the pharmacokinetic profile were observed compared to that in 9 non-elderly (age range: 21 to 31 years) males. The pharmacokinetic parameters in elderly males who received treatment with silodosin are shown in Table 3.
Table 3 Pharmacokinetic parameters following administration of a single postprandial 4 mg dose in elderly and non-elderly males (mean±SD)
| Cmax (ng/mL) | AUC0-α (ng ∙ hr/mL) | Tmax (hr) | t 1/2 (hr) | |
| Elderly male | 21.8 ± 11.6 | 142.4 ± 54.7 | 2.5 ± 1.4 | 10.5 ± 4.0 |
| Non-elderly male | 20.5 ± 6.5 | 121.5 ± 38.1 | 2.3 ± 0.5 | 8.7 ± 3.1 |
When a single 4 mg dose of silodosin was administered orally to 11 healthy adult male volunteers 30 min postprandially or under fasting conditions, the Cmax, AUC0-48hr Tmax, and t 1/2 following postprandial administration (or under fasting conditions) were 23.0 (28.0) ng/mL, 128.8 (135.9) ng∙hr/mL, 2.1 (1.4) hr, and 6.0 (4.7) hr, respectively (Table 4).
Table 4 Pharmacokinetic parameters following administration of a 4-mg dose in healthy adult male volunteers (mean±S.D)
| Cmax (ng/mL) | AUC0-48hr (ng ∙ hr/mL) | Tmax (hr) | t 1/2 (hr) | |
| Postprandial | 23.0 ± 10.8 | 128.8 ± 64.1 | 2.1 ± 0.7 | 6.0 ± 4.8 |
| Fasting | 28.0 ± 9.6 | 135.9 ± 55.4 | 1.4 ± 1.1 | 4.7 ± 3.7 |
The clearance and distribution volume following administration of silodosin solution (2 mg) to 11 healthy adult male volunteers by intravenous infusion over 4 hr were 167 .0 ± 33. 8 mL/min and 49 .5± 17.3 L, respectively. The bioavailability following a single oral administration of silodosin at a dose of 4 mg was 32.2%.
Distribution
Protein Binding
In an in vitro study, the human plasma-protein binding rate of silodosin was 95.6% (at a concentration of 100 ng/mL) and the main binding protein was α-acid glycoprotein.
Metabolism
Silodosin was metabolised mainly by CYP3A4, UGTs, ADH, and ALDH, with the major metabolites in plasma being a glucuronide and an oxidized metabolite of silodosin. When a single 8 mg dose of 14C-labeled silodosin solution was administered orally to 6 healthy male non- Japanese volunteers, the AUC0-12hr ofsilodosin and its glucuronide and oxidized metabolites relative to the AUC0-12hr of total radioactivity in plasma was 24.0, 21.9, and 34.9%, respectively. Other metabolites accounted for no more than 5%.
Excretion
In the 240-hour period after dosing, 33.5 and 54.9% of administered radioactivity was excreted in urine and feces, respectively.
The cumulative excretion in urine 0-48 hr after a single 4 mg dose of silodosin was administered orally to 12 elderly and 9 non-elderly male volunteers was 2.3 and 2.4% for silodosin, 1.6 and 1.8% for its glucuronide metabolite, and 4.5 and 4.9% for its oxidised metabolite, respectively.
Special Population
Pharmacokinetics in Patients with Lower Urinary Tract Symptoms Associated with Benign Prostatic Hyperplasia
In an exploratory population pharmacokinetic analyses (n=258) of a long-term administration study with silodosin in patients with lower urinary tract symptoms associated with benign prostatic hyperplasia, the estimated plasma concentrations of silodosin (mean±SD) at steady
state 2 and 12 hours post-dose were 24.8±8.0 and 7.4 ±3.3 ng/mL, respectively. An analysis of variable factors in relation to plasma concentrations of silodosin suggested that silodosin clearance is affected by body weight, age, CRP, ALT (GPT), and serum creatinine and distribution volume by body weight, age, CRP, and ALT (GPT). Of these factors, it was concluded that ALT (GPT) had the most effect on plasma concentrations of silodosin and it was suggested that, as a result of increased levels of ALT (GPT) (23 ➔ 83 IU/L), silodosin clearance and distribution volume may decrease by approximately 47 and 27%, respectively.
Pharmacokinetics in Patients with Impaired Renal Function
When a single 4 mg dose of silodosin was administered orally to 6 patients with impaired renal function (creatinine clearance: 27-49 mL/min) and 7 volunteers with normal renal function ( creatinine clearance: 125-176 mL/min), the total plasma concentration of silodosin was increased (Cmax: 3.1-fold; AUC0-α: 3.2-fold) in patients with impaired renal function compared to that in the volunteer group. This increase in total plasma concentration of silodosin may be attributable to protein binding with serum α1-acid glycoprotein, with a high correlation between total plasma concentration of silodosin and serum concentration of α1-acid glycoprotein observed. It should be noted that the increase in the plasma concentration of unbound silodosin (Cmax: 1.5-fold; AUC0-α: 2.0-fold), which is considered to have a direct bearing on the manifestation of drug effect and incidence of adverse reactions associated with silodosin, was less than that for the total drug concentration (Table 5).
Table 5 Pharmacokinetic parameters following administration of a single 4 mg dose in patients with impaired renal function and volunteers with normal renal function under fasting conditions (mean±SD).
| Cmax (ng/mL) | AUC0-α (ng ∙ hr/mL) | Tmax (hr) | t 1/2 (hr) | |
| Impaired renal function | 72.22±44.12 (1.48±1.30) | 305.76±115.38 (6.34±3.43) | 0.67±0.26 (0.83+0.26) | 7.55±1.50 (8.71 ±3.94) |
| Normal renal function | 21.51±8.52 (0.71±0.13) | 94.75±41.28 (2.96±1.09) | 0.86±0.56 (0.86±0.56) | 3.94±1.57 (4.39±1.34) |
| Values in ( ) indicate plasma concentration of unbound silodosin. | ||||
Other details
Drug Interactions
Non-Japanese data
Ketoconazole (oral preparation not marketed in Japan) coadministration
When 16 healthy male volunteers (non-Japanese) who were receiving 200 mg of ketoconazole (p.o.) once daily for 4 days were coadministered a single 4 mg dose of silodosin (p.o.) on day 2, Cmax and AUC0-α of silodosin increased 3.7- and 2.9-fold, respectively, compared to when silodosin alone was administered.
Digoxin coadministration
When silodosin (4 mg, twice daily) was coadministered orally with digoxin (0.25 mg, once daily) for 8 days to 16 healthy male volunteers (non-Japanese), it was confirmed that silodosin has no effect on the pharmacokinetic profile of digoxin.

